Vaccine Update: Importance of Provider Recommendation for RSV Protection This Viral Season
Column Author: Yvonne M Carter, MSN, APRN II, FNP-C
Column Editor: Angela L. Myers, MD, MPH | Division Director, Infectious Diseases; Medical Director, Center for Wellbeing
As we prepare for another busy respiratory virus season, it is important to review preventive measures for patients and families against some of the most common and contagious respiratory viruses. Last month’s Link issue covered the updated 2024 recommendations for influenza and COVID-19 vaccines. When it comes to respiratory syncytial virus (RSV), this year marks the second in history that providers have had the opportunity to protect infants by offering either maternal RSV vaccination (Abrysvo) or long-acting RSV monoclonal antibody (nirsevimab). Abrysvo is recommended for pregnant individuals at 32 to 36 weeks’ gestation to prevent lower respiratory tract disease (LRTD) and severe LRTD from RSV in infants from birth to age 6 months. Nirsevimab is currently recommended to infants <8 months of age born during, or entering, their first RSV season – unless they were born 14 or more days after their mother received Abrysvo. Nirsevimab is also recommended for certain infants and toddlers aged 8-19 months at increased risk for severe RSV who are entering their second RSV season.1,2
RSV has long been one of the most common causes of hospitalization for U.S. infants and young children. While the Centers for Disease Control and Prevention (CDC) currently projects this season’s RSV hospitalization rates across all age groups to be similar to or lower than that of the 2023-2024 season, this projection is largely due to expectations that new RSV vaccines and immunizations will play an important role in preventing hospital admissions due to RSV. With assumed RSV immunization effectiveness of 90% (nirsevimab) and maternal vaccination effectiveness of 64% (Abrysvo), recent CDC modeling indicates that 43%-76% of hospitalizations of infants would be prevented with immunization uptake (with 43% prevented with low uptake and up to 76% prevented with high uptake), compared to a scenario without any immunization.3
In the recent Sept. 26 issue of the CDC’s Morbidity and Mortality Weekly Report (MMWR), authors discussed the importance of provider recommendation when it comes to uptake of both maternal RSV vaccination and infant RSV immunization. In efforts to determine maternal and infant RSV immunization coverage from 2023 to 2024, the CDC conducted an internet panel survey from March 26 to April 11, 2024. Of 678 pregnant mothers at 32-36 weeks’ gestation from September 2023 to January 2024, 32.6% of women reported receiving an RSV vaccine during pregnancy. And of 866 women with an infant born during August 2023-March 2024, 44.6% reported their infant received nirsevimab. Between the two methods, 55.8% of infants from mothers surveyed were protected by maternal RSV vaccine, nirsevimab, or both. The survey found one barrier to RSV immunization was concern about long-term safety for the infant. Both nirsevimab and Abrysvo were new to the market last season. However, lack of provider recommendation was cited as the main reason for not getting RSV immunization, and provider recommendation was associated with higher immunization coverage for both maternal RSV vaccination and nirsevimab receipt.4 A graphic looking at monthly nirsevimab receipt and intent among pregnant females throughout the 2023-2024 season revealed that in some months “probable” intent or uncertainty was greater than “definite” intent to get nirsevimab for the baby (Figure 1). This finding calls attention to the significant difference providers can make by engaging patients and families in key conversations.
Figure 1. Monthly Nirsevimab Receipt and Intent Among Females Aged 18-49 Years Who Have an Infant < 8 Months, Are Currently Pregnant, or Are Trying to Get Pregnant, United States
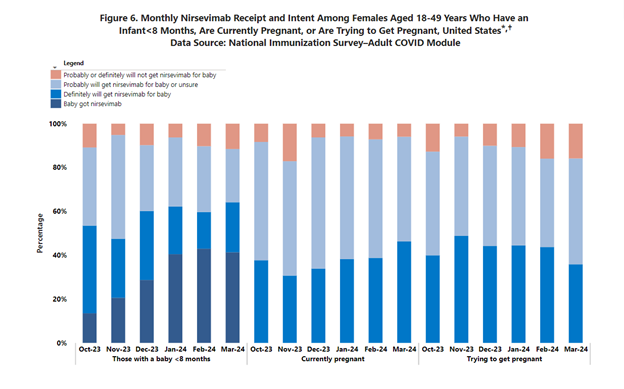
The CDC survey highlights the important and influential role providers play in ensuring protection against RSV for our most vulnerable patients. Institutions should empower and educate providers to have these crucial discussions with patients and families, or at the very least explore the topic of RSV immunization with them. Simply put, any activities that support providers to have these important conversations with patients may directly translate to higher rates of immunization against RSV and lead to lower rates of hospitalization for RSV. Motivational interviewing is an evidence-based technique that has been studied by health care providers in other interventions, such as COVID-19 vaccine discussions. However, the same techniques may also be used this 2024-2025 respiratory virus season to address RSV immunization.5 Additional immunization standards for adults (that can be used in addressing vaccines in pregnancy as well as for parents and their children) can be found on the CDC website here.6 The American College of Obstetricians and Gynecologists also has resources to aid providers in communicating the importance of immunization for pregnant people.7 All in all, it is better to address the issue than not. Moms and babies are counting on us.
References:
- RSV vaccine guidance for pregnant people. Centers for Disease Control and Prevention. August 30, 2024. Accessed October 9, 2024. https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/pregnant-people.html
- Healthcare providers: RSV immunization for infants and young children. Centers for Disease Control and Prevention. Last reviewed September 28, 2023. Accessed October 9, 2024. https://www.cdc.gov/vaccines/vpd/rsv/hcp/child.html
- 2024-2025 respiratory disease season outlook. Centers for Disease Control and Prevention. August 29, 2024. Accessed October 9, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/season-outlook24-25/
- Razzaghi H, Garacci E, Kahn KE, et al. Maternal respiratory syncytial virus vaccination and receipt of respiratory syncytial virus antibody (nirsevimab) by infants aged < 8 Months–United States, April 2024. MMWR Morb Mortal Wkly Rep. 2024;73(38):837-843. Accessed October 2, 2024. https://www.cdc.gov/mmwr/volumes/73/wr/mm7338a2.htm?s_cid=mm7338a2_w
- Vaccines and immunizations: talking with patients about COVID-19 Vaccination. Centers for Disease Control and Prevention. Last reviewed November 3, 2021. Accessed October 9, 2024. https://www.cdc.gov/vaccines/covid-19/hcp/engaging-patients.html
- Adult immunization standards. Centers for Disease Control and Prevention. August 9, 2024. Accessed October 9, 2024. https://www.cdc.gov/vaccines-adults/hcp/imz-standards/
- Maternal RSV vaccination. The American College of Obstetricians and Gynecologists. Accessed October 9, 2024. https://www.acog.org/clinical-information/physician-faqs/maternal-rsv-vaccination
